H2CO3 H2O CO2 is decomposition reaction More. Fortunately there are not that many types of reaction.
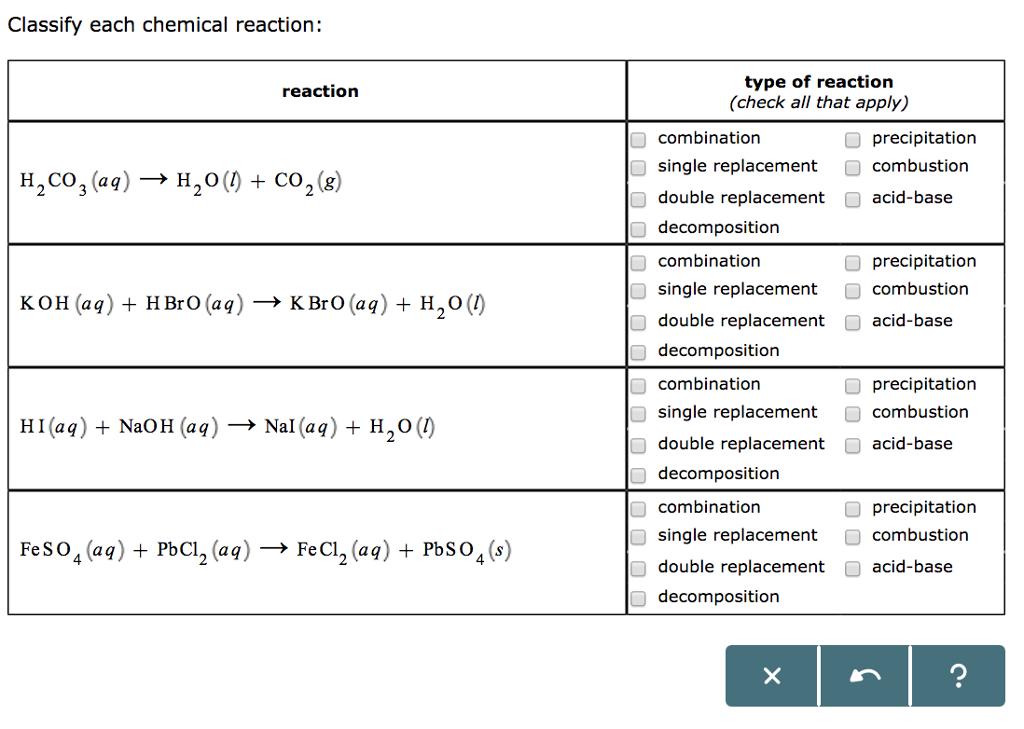
Solved Classify Each Chemical Reaction Type Of Reaction Chegg Com
Carbonic acid H2CO3 a compound of the elements hydrogen carbon and oxygen.
. In order to complete chemical reactions you must be able to write formulas for ionic compounds. HF 2 H2O CaF2 CaF2 precipitates 2 2. D The lone pairs in the H2O molecule are used for forma new bond with the carbon in the CO2 molecule.
The oxidizing agent. Give reasons for the products. See link At neutral pH the uncatalyzed hydration-dehydration of CO2 by reactions 1 or 2 is a slow process having the following approximate rate constants at 25C.
Calcium carbonate is not very soluble in water. For each oxidation-reduction in Question 2 identify the. Calcium Carbonate Hydrogen Chloride Calcium Chloride Water Carbon Dioxide.
Up to 24 cash back Types of Chemical Reactions Answers Balance each of the following reactions and identify each type of reaction. Hence we can classify chemical reactions according to the type of reaction. It undergoes partial dissociation in the presence of water to yield H and bicarbonate ions.
2 NH 3 H 2 SO 4 NH 4 2 SO 4 synthesis 3. Up to 24 cash back C2H8 4 O2 2 CO2 4 H2O combustion. H2CO3 aq H2O l CO2 g.
Up to 24 cash back If the word equation is incomplete complete it and write the balanced chemical equation. Carbonic Acid H2CO3 -Carbonic acid is a chemical compound with the formula H2CO3. Identify the type reaction from the following.
K K eq no special nomenclature 3. Along the way we examine various aspects of reactions. CaCO 3 is a base HCl is an acid.
In this video we determine the type of chemical reaction for the equation H2CO3 H2O CO2 Carbonic acid breaking downSince we one substance breaking a. B H2CO3 forms a coordinate covalent bond by a Lewis acid base reaction. To learn more about Structure Properties Preparation Uses and FAQs of Carbonic Acid.
In this chapter we will begin with combustion reactions and end with oxidationreduction reactions. Reaction Types Use these reaction types to answer the following questions. There are many classifications possible and one reaction may be classified in more than one way.
Na2CO3 H2O CO2 H2O H2CO3. Predict the products determine if it will react and balance. Li 3 N 3.
All combustion reactions fall into the pattern. 4 C 5 H 9 O 29 O 2 20 CO 2 18 H 2 O combustion 4. BaOH 2 aq Na 2 CO 3.
An acid and a base react with each other. H2CO3 NaOH Na2CO3. Carbonic acid can be considered to be a diprotic acid from which two series of salts can be formednamely.
H2CO 3 aq HCO 3-aq H An acid dissociation reaction. Visit BYJUs for detailed information. K13710-2s-l k-114s-1 k485103litermol-1s-l and k-4210-4s-1.
3 Pb 2 H 3 PO 4 3 H 2 Pb 3 PO 4 2 single displacement 5. Types of Chemical Reactions Worksheet. CHEM 1105 CLASSIFICATION OF REACTIONS There are very many chemical reactions known.
This is a bond reorganization reaction. Identify the type of chemical reaction. Tell the type of reaction.
For each of the following reactions. A CO2 would be called a bronsted lowry acid and H2O would be called a bronsted Lowry base. 2 NaBr CaOH 2 CaBr 2 2 NaOH double displacement 2.
Remember to write the formula for ionic compounds all you have to do is criss-cross the numerical value of the charges into subscripts for the elements. CO2 H2O H2CO3 The predominant species are simply loosely hydrated CO2 molecules. There are many types of chemical reactions.
C CO2 would be called a Lewis acid and H2O would be called Lewis base. This is an acid-base reaction neutralization. H2CO3 - H2O CO2.
It is formed in small amounts when its anhydride carbon dioxide CO2 dissolves in water. CO2 H2O Cl2 LiBr calcium. Rate constant of the reaction H2CO3H2OCO2 in the absence of carbonic anhydrase.
CaCO3 2HCl CaCl2 H2O CO2. It is a weak acid. Classify the following reaction by giving all of the reaction types that apply.
HCO 3-aq CO 3. Generally the product of this reaction is a salt and. Commonly given equilibrium constant notations of Ka if it is a monoprotic acid or K a1 for the first acid dissociation of a polyprotic acid 4.
CaCO3 HCl CaCl2 H2O CO2. It is also a type of hydration reaction with water. H2CO3H2O CO2 reversible decomposition synthesis exchange Weegy.
Identify the type of chemical reaction.
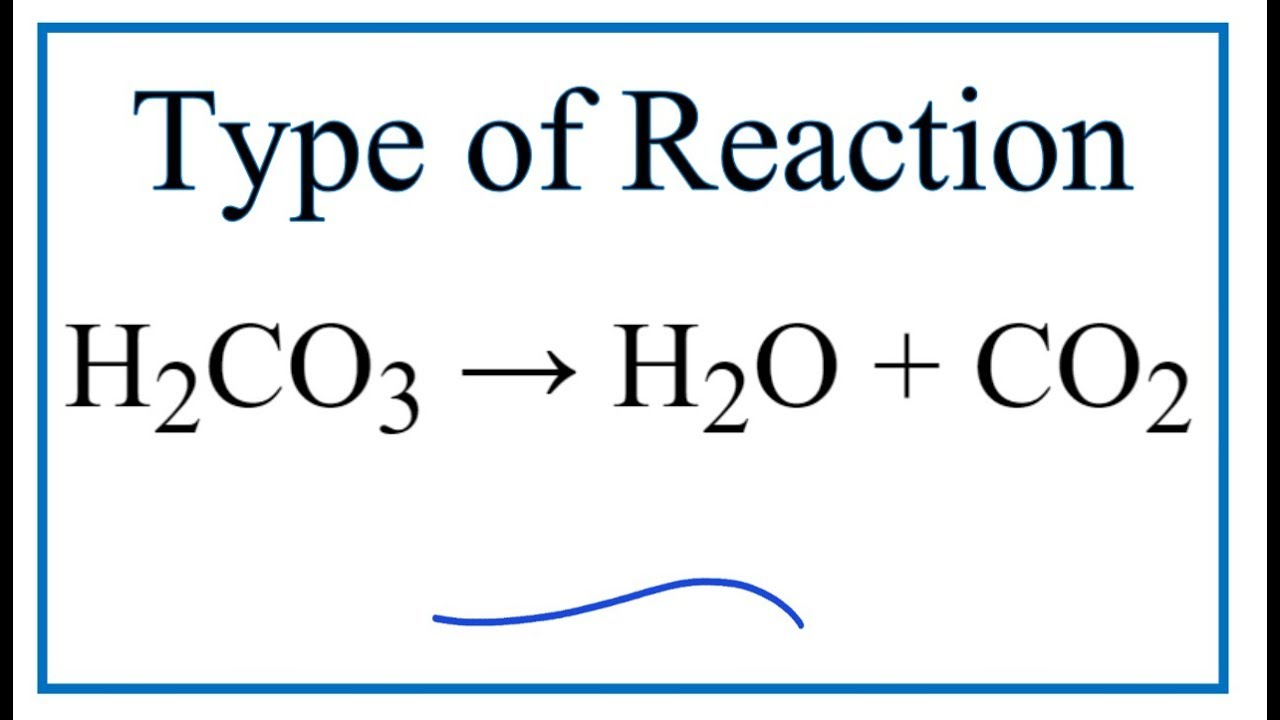
Type Of Reaction For H2co3 H2o Co2 Youtube

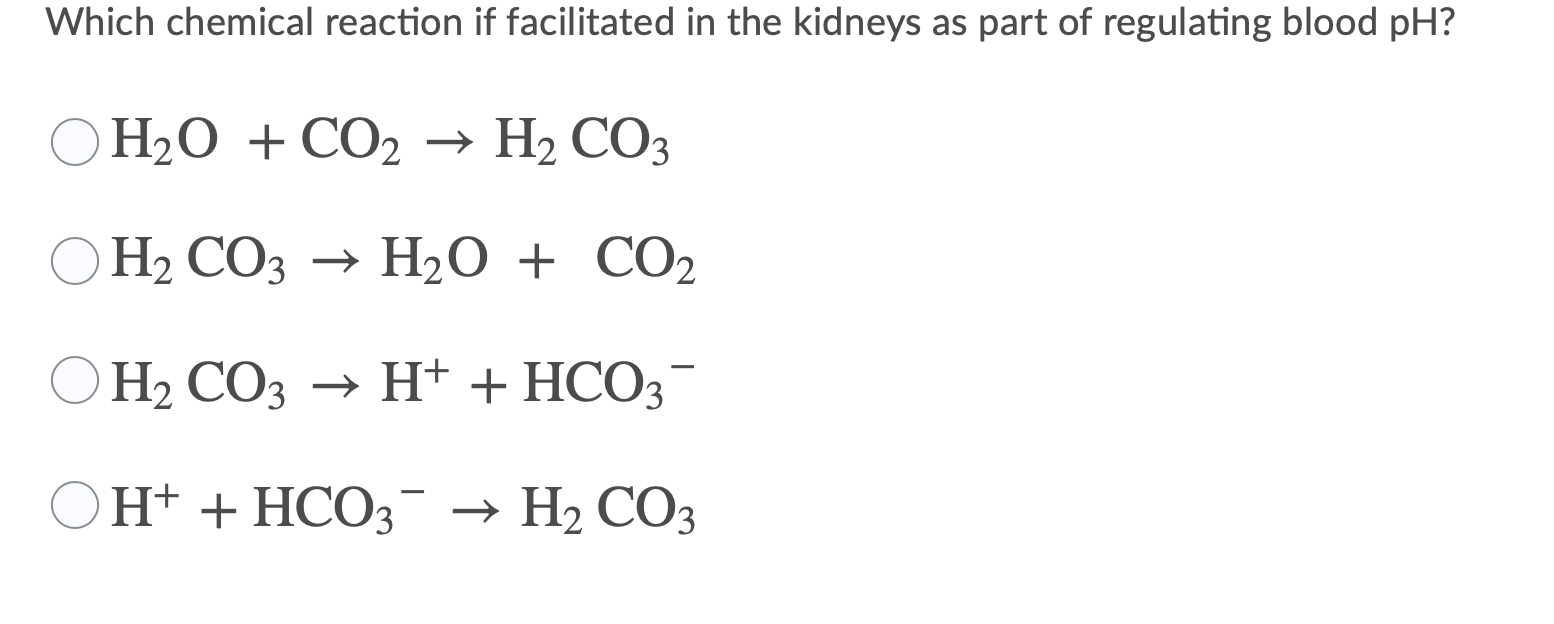
Solved Which Chemical Reaction If Facilitated In The Kidneys Chegg Com

How To Balance H2co3 H2o Co2 Decomposition Of Carbonic Acid Youtube
0 Comments